
CDMO
我公司拥有水针制剂及固体制剂的委托加工(CDMO)平台。公司可提供包括药品研发、中试放大、工艺验证、商业化生产等“一站式”服务。
KIVIPharm has a CDMO platform for water injection and oral solid dosage. Our company canprovide "one-stop" services including drug development, pilot scale-up, process validation, and commercial production.

凯威CDMO优势:
Our strengths:
1、水针车间配备专利级全无菌灌装系统
SVP plant has a patented full aseptic filling system
2、水针车间可生产微含量小水针制剂
SVP plant can
produce SVPs of tiny
content
3、高附加值的固体制剂生产车间
Oral solid
plant can produce high value-added oral solids
4、公斤级的API生产车间
APIs plant can
produce APIs within 20kg
小容量注射剂车间
公司有近40年的专业小容量安瓿瓶水针注射剂的生产经验。水针车间拥有三条最终灭菌生产线和一条非最终灭菌生产线,可生产从1ml-20ml规格不等的各种小容量注射剂。车间生产线采用国内先进的楚天联动线,注射用水采用RO2+EDI系统,生产能力为每小时8吨。新配置的专利级全无菌制剂灌装系统可有效保证制剂真正意义上无菌稳定生产。
Our SVP Plant has 40 years of operational
excellence. SVP products present in more than 50 countries. The SVP Plant has
three terminal sterilizing lines and one non-terminal sterilizing line. The
strength of the volume of our products ranging from 1ml to 20ml. The workshop production line adopts the most
advanced Truking Automatic Inspection linkage line in China. RO2+EDI is used
for an injection water system which capacity is 8 tons per hour. Patented full
aseptic filling system which can effectively ensure aseptic and stable
production of the preparation.
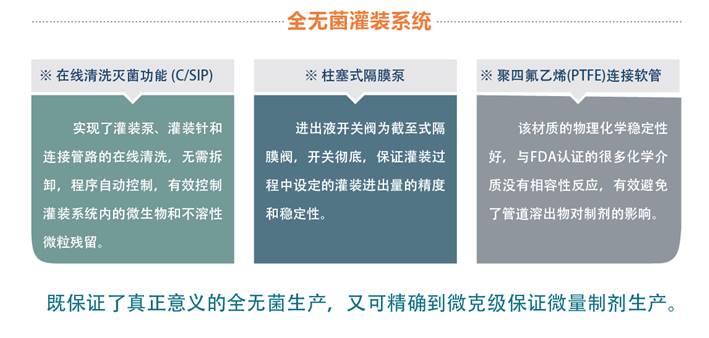
※在线清洗灭菌功能 (C/SIP)
Online cleaning and
sterilization function
实现了灌装泵、灌装针和连接管路的在线清洗,无需拆卸,程序自动控制。有效控制灌装系统内的微生物和不溶性微粒残留。
The filling pump, filling needle and
connecting pipes can be cleaned on-line without disassembly. The system can control
automatic and it also can control the vestigital of microorganism and insoluble particle
effectively
※柱塞式隔膜泵
Plunger
Diaphragm Pump
进出液开关阀为截至式隔膜阀,开关彻底,保证灌装过程中设定的灌装进出量的精度和稳定性。
The switch of the pump can control the flow
of the liquid accurately. so, the stability and precision of the filling volume
can be well controlled
※聚四氟乙烯(PTFE)连接软管
PTFE
Connecting Pipes
该材质的物理、化学稳定性好,与FDA认证的很多化学介质没有相容性反应。有效避免了管道溶出物对制剂的影响。
The physical and chemical stability of the
material of the pipes is good. There is no compatible reaction with the medium
which were certificated by FDA. It can effectively avoid the influence of pipe
dissolution on the preparation.
水针车间不仅可以保证制剂的全无菌生产,而且可以做到精确到微克级的高活性低微量制剂生产。
In brief, we can guarantee both full aseptic
production and accurate preparation to the microgram level
固体制剂车间
Camfield—OSD Plant
OSD车间按照FDA和欧盟标准设计建设,设备选用国内先进的山东新马产品。可实现湿法制粒、干燥、制微丸(预留)、干法制粒、总混、压片(含微片)、包衣、胶囊充填、颗粒剂(预留)、内包(瓶装/铝塑/铝铝)、外包等功能;定位为规模化品种中试和贵细药品的生产。
The new OSD workshop includes the first and
second phase production lines. The first phase line is for pilot scale-up
products or expensive & small size products, which can achieve
multi-functions such as wet & dry granulation, drying, mini-pills (for
future use), blending, tablets compression (mini-tablets included), coating,
capsules filling, granules (for future use), primary & secondary packaging
(bottling/Al-Plastic/Al-Al) etc.. the annual production capacity is 300 million
tablets/capsules.

口服固体CMO及CDMO优势:
1、完善的质量体系,为产品质量提供可靠保证
公司质量保证系统按照美国、欧盟等先进GMP标准搭建。目前固体制剂片剂和胶囊剂产品已申报FDA和欧盟认证,并通过欧盟QP质量审计。
2、先进的设计理念及一流生产设备,为产品质量提供强劲支持
厂房设施采用美国FDA和欧盟EMA先进GMP标准设计建造,生产设备国内一流厂家购置,可实现设备操作PLC控制、自动取样、自动剔除、异常报警等功能,自动化程度高
3、优秀的企业理念和高素质的员工队伍,是产品质量的基础
企业方针:以人为本、服务优先、品质创新、引领健康
企业愿景:国际化、专业化的药品生产企业
企业使命:优质药品,健康生活
固体制剂可承接湿法制粒、干法制粒、干法直压的片剂、胶囊剂、颗粒剂(预留)及微丸产品的生产。
原料药车间
Camfield—API Plant
原料药车间有南、北、东三条生产线,其中北线和东线按照欧盟和FDA标准建立,能生产几克至二十公斤批量的API;南线能进行普通化学中间体合成。
API Plant has three product line located respectively
on first, second, and third line. The first and second line were established according
to EU and FDA requirements. The third line can accomplish the synthesis of
ordinary chemical intermediates. Our API Plant can produce APIs within A few
grams to twenty kilos.

CDMO业务:
联系电话:0313-5906965
范经理(Manager Fan):15613186982


